International trade
Creating trade policy for a life sciences superpower
The UK is a life sciences superpower. Its exceptional strength in the research and production of world-class medicines and vaccines has developed over many decades out of a strong science base, open approach to foreign investment in pharmaceuticals, a skilled manufacturing workforce, and robust culture of protecting innovation through intellectual property (IP) rights. Its regulators are recognised as among the most experienced and sophisticated in the world.
This ecosystem enables the UK pharmaceutical sector to thrive as a major exporter of life-saving medicines to patients across the globe. Over £26.1 billion worth of medicinal and pharmaceutical products were exported around the world in 20231 – the third highest goods sector in the economy, which has increased 8% in the past 5 years2. This figure is almost double the £14 billion worth of branded medicines procured by NHS England in 20233, illustrating the scale and importance of UK pharmaceuticals trade flows compared to domestic consumption.
£25.4 billion worth of medicinal and pharmaceutical products were imported in 20234, meaning a trade surplus of £700 million in medicinal and pharmaceutical products.
As well as exporting vital medical goods, the UK also exports life sciences ideas – licensing treatments for production, converting its own IP into manufactured medicines in markets around the world, and shaping debates about the present and future of medicines regulation and pharmaceutical innovation.
The sector’s strength is already recognised in the Life Sciences Industrial Strategy, where the Government has laid out its vision for transforming the UK into a global life sciences hub.
Outside of the EU, the UK needs a trade policy that aligns with this goal and is fully customised to its unique features: enabling the sector to pull in the imports, talent, and capital it needs for world-class science and manufacturing, and ensuring it can export UK pharmaceutical innovation easily and compete fairly in export markets. A trade policy for the pharmaceutical sector can and should encompass all these aspects in a single strategic vision that supports the Government’s broader industrial policy and public service priorities.
This vision can then inform a consistent UK approach to Free Trade Agreements (FTAs) and wider trade policy.
To achieve the Government’s ambition for the UK to thrive as a life sciences superpower, the Association of the British Pharmaceutical Industry (ABPI) believes the Government should establish three core pillars for its trade policy for pharmaceuticals.
References:
[The following data was verified on 17 July 2024]
- Trade in Goods: Country-by-commodity exports, Office of National Statistics [Accessed 17 July 2024]
- UK trade: May 2024, Office for National Statistics [Accessed 17 July 2024]
- Aggregate net sales and payment information: February 2024, DHSC [Accessed 17 July 2024]
- Trade in Goods: Country-by-commodity imports, Office for National Statistics [Accessed 17 July 2024]
Growing Britain’s life sciences sector through international and trade policy
In a changing international context, the UK needs a new approach to international and trade policy for the life sciences.
The sector is strategically important to the domestic economy and global health security and has been rightly identified by the new government as key to delivering its core missions.

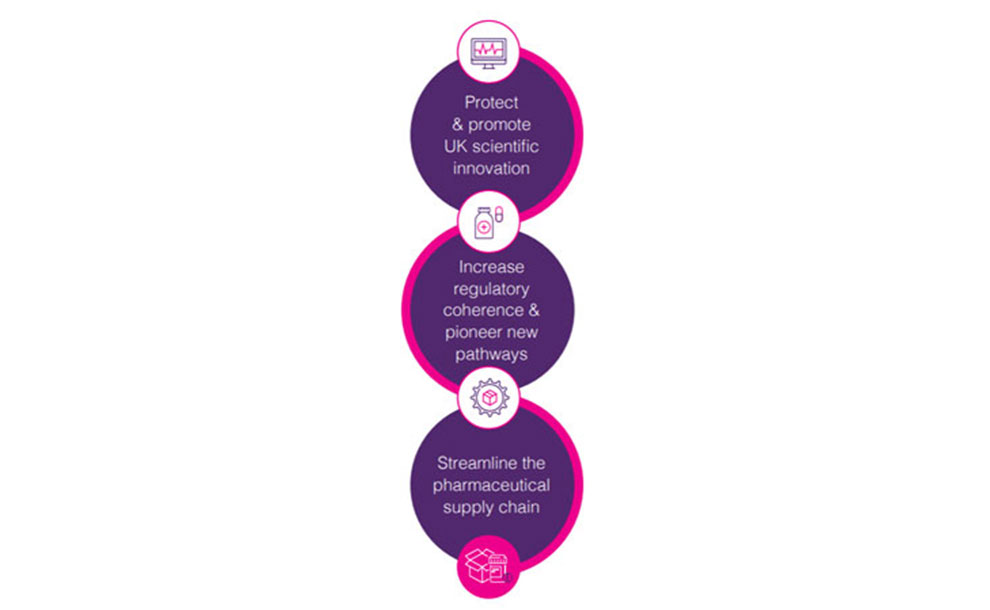
A life sciences superpower:
3 key benefits for the UK
- Companies will continue to invest in pharmaceutical R&D and manufacturing in the UK, creating highly skilled and productive jobs across the entire country.
- The removal of tariff and non-tariff barriers will boost high-value UK exports.
- Streamlined supply chains and regulatory practices support the UK’s public service priorities by helping to deliver the best possible treatments and value to the NHS.
A strategic trade policy for the UK pharmaceutical sector: a summary of ABPI recommendations
UK scientific innovation
- Develop an ‘IP diplomacy’ strategy to promote UK standards in export markets.
- Ensure that preferential trading partners commit to a clear baseline of best practice for IP that reflects current UK standards.
- Ensure that pricing and reimbursement processes are fair, transparent and do not discriminate against UK companies
Medicines regulation and policy
- Encourage all countries to regulate medicines to the highest international standards.
- Develop and deepen formal channels of cooperation on medicines regulation to remove duplicative processes.
- Work collaboratively with regulatory peers to pioneer new thinking behind novel regulatory pathways.
The pharmaceutical supply chain
- Ensure the full elimination of all tariffs on finished medicines, intermediaries, and raw materials, beyond those listed by the WTO Pharma Agreement & Annex.
- Adopt a simplified, common approach to origin requirements and customs protocols for all UK FTAs.
No FTA commitments should in any way limit the UK’s freedom to define the policy of the NHS as it wishes, in line with its existing international commitments to non-discrimination.
Trade policy toolkit
The ABPI sees four basic tools available to promote and champion a trade policy for the UK pharmaceutical sector.
A strategy built around these pillars utilising these tools can reinforce the role of the UK as a global platform for developing, manufacturing, and exporting world-class pharmaceuticals. The next three sections of this ABPI report set out a strategic vision for UK trade policy for each of these pillars
FTA negotiations
FTAs are an important tool for aligning and locking in common high standards in the UK and with any trading partner.
They can provide a joint commitment to robust IP protection, tariff liberalisation, openness to investment and effective medicines regulation, as well as commitments on innovation, by binding best practice in both markets and securing opportunities for further collaboration, which enhances the value of export markets.
The WTO framework
The WTO framework has several agreements and mechanisms specifically targeted to trade in pharmaceuticals that urgently need updating to reflect technological change and medical advancement – such as the WTO Pharmaceutical Tariff Elimination Agreement.
The UK can and should advocate for these changes and encourage adoption of, and adherence to, important international rules on public procurement, technical regulation of pharmaceuticals, and IP.
Regulatory diplomacy
As a highly regulated sector, the conditions of medicines regulation in export markets are a key determinant of UK export success. Strong links between UK regulators and their peers can support convergence in regulatory practice, aligning approaches to emerging regulatory questions and formally recognising UK ‘gold-standard’ approaches, or those similar in partner countries, in a way that reduces duplicative processes for exporters.
Unilateral policy in the UK
There are a range of choices the UK can make for its own framework that will support its performance as a life sciences superpower. Outside of the EU, the UK can set its general external tariff at the appropriate level to ensure competitive costs for manufacturing inputs and continue to enhance its robust framework for protecting IP to enable timely patient access to new medicines in the world.
Facilitating trade in health products through international manufacturing recognition and harmonisation policy
The UK can bolster its position as a leader in life sciences by charting a pragmatic approach towards, and engaging purposefully with, a global recognition policy for manufacturing.


WTO Twelfth Ministerial Conference: A Critical Opportunity to Strengthen the Global Trade and Health Agenda
The WTO’s Twelfth Ministerial Conference (MC12) presents an opportunity for WTO members to formalize and pursue a robust trade and health agenda that builds on these principles and further strengthens the open and rules-based international trading system in response to COVID-19 and future health crises.
Related content in this section:
Last modified: 28 September 2023
Last reviewed: 28 September 2023